Electrochemistry Communications Journal - Elsevier Electrochemistry involves the study of the relationship between electrical signals and chemical systems that are incorporated into an electrochemical cell. It plays a very important role in many areas of chemistry, including analysis, thermodynamic studies, synthesis, kinetic measurements, energy conversion, and biological electron transport [ 1 ].
Lecture Notes Electrochemical Energy Systems Chemical
electrochem Typ (1).pdf Google Drive. • Electrochemistry is the study of the relationship between chemical change and electrical work. • It is examined via the use of electrochemical cells which are systems that incorporate a redox reaction to produce or utilize electrical energy. • Isolated oxidation and reduction processes are not much good. These reactions must be, and interdisciplinary nature of electrochemistry, and particularly of electrode reactions, through a description of modern electrochemistry. Secondly to show to the student and the non-specialist that this subject is not separated from the rest of chemistry, and how he or she can use it..
Electrochemistry Basics Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as an oxidation-reduction ("redox") reaction. Electrochemistry by Mohammed A. A. Khalid. Electrochemistry has been undergoing significant transformations in the last few decades. It is now the province of academics interested only in measuring thermodynamic properties of solutions and of industrialists using electrolysis or manufacturing batteries, with a huge gap between them.
Electrochemistry grew out of the same tradition which gave physics the study of electricity and magnetism. The reputed founders of physical chemistry-Arrhenius, Ostwald, and van't Hoff-made many of their contributions in areas which would now be regarded as electrochemistry. With the post-World War II capture of physical chemistry by chemical Fundamentals of Electrochemistry provides the basic outline of most topics of theoretical and applied electrochemistry for students not yet familiar with this field, as well as an outline of recent and advanced developments in electrochemistry for people who are already dealing with electrochemical problems. The content of this edition is
Read and learn for free about the following article: Electrochemistry If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Text book of electrochemistry (PDF 364P) This note covers the following topics: Fundamental Physical and Chemical Conceptions, Older Electrochemical Views, The Laws of Avogadro and van't Hoff, Vapour Pressure of Solutions, Boiling Point and Freezing Point of Solutions, General Conditions of Equilibrium, Velocity of Reaction, Electrolytes
Electrochemistry by Mohammed A. A. Khalid. Electrochemistry has been undergoing significant transformations in the last few decades. It is now the province of academics interested only in measuring thermodynamic properties of solutions and of industrialists using electrolysis or manufacturing batteries, with a huge gap between them. Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
Chemistry Notes for class 12 Chapter 3 Electrochemistry Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry 1 Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
Electrochemistry is the study of reactions in which charged particles (ions or electrons) cross the interface between two phases of matter, typically a metallic phase (the electrode) and a conductive solution, or electrolyte. A process of this kind can always be represented as a chemical reaction and is known generally as an electrode 5_ Electrochemistry.pdf - Read online for free. Much more than documents. Discover everything Scribd has to offer, including books and audiobooks from major publishers.
Téléchargez l ebook Fundamentals of Electrochemistry, Vladimir S. Bagotsky - au format ePub pour liseuse, tablette, smartphone ou ordinateur: PDF. (New version, 2018) The eight lessons in this section of the Chem1 Virtual Textbook cover elementary electrochemistry in somewhat greater depth than is found in standard textbooks, but at a level still suitable for first-year college and advanced high school courses.
electrochemistry which is our first real example of modern analytical chemistry. By that we mean that plenty of scientists do electrochemistry today because it is often the best way to solve certain problems in chemical analysis like understanding corrosion (rust). Electrochemistry involves the study of the relationship between electrical signals and chemical systems that are incorporated into an electrochemical cell. It plays a very important role in many areas of chemistry, including analysis, thermodynamic studies, synthesis, kinetic measurements, energy conversion, and biological electron transport [ 1 ].
13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer Téléchargez l ebook Fundamentals of Electrochemistry, Vladimir S. Bagotsky - au format ePub pour liseuse, tablette, smartphone ou ordinateur: PDF.
Téléchargez l ebook Fundamentals of Electrochemistry, Vladimir S. Bagotsky - au format ePub pour liseuse, tablette, smartphone ou ordinateur: PDF. 13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer
Electrochemistry (article) Khan Academy
Lecture Notes Electrochemical Energy Systems Chemical. Free PDF download of Class 12 Chemistry revision notes & short key-notes for Chapter 3 - Electrochemistry to score high marks in exams, prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books., electrochemistry, beginning with techniques and passing through mechan isms to electroanalytical chemistry, synthesis, industrial electrochemistry, batteries and finally corrosion. All prefaces end with the author thanking a spouse. This is not mere form. Academics with ….
Lecture Notes Electrochemical Energy Systems Chemical. Text book of electrochemistry (PDF 364P) This note covers the following topics: Fundamental Physical and Chemical Conceptions, Older Electrochemical Views, The Laws of Avogadro and van't Hoff, Vapour Pressure of Solutions, Boiling Point and Freezing Point of Solutions, General Conditions of Equilibrium, Velocity of Reaction, Electrolytes, 13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer.
Fundamentals of Electrochemistry Google Books
Electrochemistry Review YouTube. 25/08/2015 · Everything you need to know about Electrochemistry. Electrochemistry is the relationship between electricity and chemical reactions. There are two ways that https://en.wikipedia.org/wiki/International_Society_of_Electrochemistry Electrochemistry involves the study of the relationship between electrical signals and chemical systems that are incorporated into an electrochemical cell. It plays a very important role in many areas of chemistry, including analysis, thermodynamic studies, synthesis, kinetic measurements, energy conversion, and biological electron transport [ 1 ]..

Electrochemistry is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice versa. Electrochemistry is the study of reactions in which charged particles (ions or electrons) cross the interface between two phases of matter, typically a metallic phase (the electrode) and a conductive solution, or electrolyte. A process of this kind can always be represented as a chemical reaction and is known generally as an electrode
Free PDF download of Class 12 Chemistry revision notes & short key-notes for Chapter 3 - Electrochemistry to score high marks in exams, prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books. Téléchargez l ebook Fundamentals of Electrochemistry, Vladimir S. Bagotsky - au format ePub pour liseuse, tablette, smartphone ou ordinateur: PDF.
13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter- relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1 . CONDUCTORS AND NON
Electrochemistry by Mohammed A. A. Khalid. Electrochemistry has been undergoing significant transformations in the last few decades. It is now the province of academics interested only in measuring thermodynamic properties of solutions and of industrialists using electrolysis or manufacturing batteries, with a huge gap between them. For more information on the wondrous properties of electrochemistry, check out our electrochemistry PDF below. Download Electrochemistry PDF; To learn more about important concepts related to electrochemistry, such as the Nernst equation, download BYJU’S – The Learning App.
Electrochemistry is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice versa. Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
Fundamentals of Electrochemistry provides the basic outline of most topics of theoretical and applied electrochemistry for students not yet familiar with this field, as well as an outline of recent and advanced developments in electrochemistry for people who are already dealing with electrochemical problems. 01/10/2017 · Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter-relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1. CONDUCTORS AND NON
Téléchargez l ebook Fundamentals of Electrochemistry, Vladimir S. Bagotsky - au format ePub pour liseuse, tablette, smartphone ou ordinateur: PDF. Fundamentals of Electrochemistry provides the basic outline of most topics of theoretical and applied electrochemistry for students not yet familiar with this field, as well as an outline of recent and advanced developments in electrochemistry for people who are already dealing with electrochemical problems.
Electrochemistry Notes Calculating Electrochemical Potential of a Cell 1) Write two ½ reactions written as reductions 2) Compare E’s––the reaction with the highest E is reduced, the other is oxidized Fundamentals of Electrochemistry provides the basic outline of most topics of theoretical and applied electrochemistry for students not yet familiar with this field, as well as an outline of recent and advanced developments in electrochemistry for people who are already dealing with electrochemical problems.
Read and learn for free about the following article: Electrochemistry If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Electrochemistry Basics Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as an oxidation-reduction ("redox") reaction.
13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer Chemistry Notes for class 12 Chapter 3 Electrochemistry Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry 1
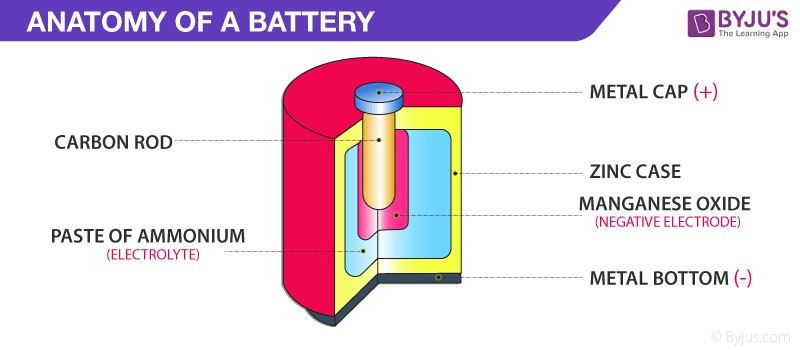
electrochemistry, beginning with techniques and passing through mechan isms to electroanalytical chemistry, synthesis, industrial electrochemistry, batteries and finally corrosion. All prefaces end with the author thanking a spouse. This is not mere form. Academics with … Electrochemistry is the study of reactions in which charged particles (ions or electrons) cross the interface between two phases of matter, typically a metallic phase (the electrode) and a conductive solution, or electrolyte. A process of this kind can always be represented as a chemical reaction and is known generally as an electrode
Electrochemistry questions (practice) Khan Academy

Electrochemistry Review YouTube. Free PDF download of Class 12 Chemistry revision notes & short key-notes for Chapter 3 - Electrochemistry to score high marks in exams, prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books., Electrochemistry Online Quiz Tnpsc Questions Study Materials PDF PPT are listed in details,most of the question have been asked in Group 1 2 2a 4 Exams..
Electrochemistry Online Quiz TNpsc Study Material Group 1
Electrochemistry Basics Chemistry LibreTexts. Fundamentals of Electrochemistry provides the basic outline of most topics of theoretical and applied electrochemistry for students not yet familiar with this field, as well as an outline of recent and advanced developments in electrochemistry for people who are already dealing with electrochemical problems., Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication..
Fundamentals of Electrochemistry: Lecture 1 5/7/03 6 5/7/03 Junction Potentials • In any circuit there are junction potentials whenever two dissimilar materials come into contact. • We focus on the metal-solution interface in electrochemistry 5/7/03 Electrochemical Thermodynamics Every substance has a unique propensity to contribute to a Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
Electrochemistry has been undergoing significant transformations in the last few decades. It is now the province of academics interested only in measuring thermodynamic properties of solutions and of industrialists using electrolysis or manufacturing batteries, with a huge gap between them. Fundamentals of Electrochemistry pdf. Fundamentals of Electrochemistry pdf : Pages 719. Second Edition by V. S. BAGOTSKY
Chemistry Notes for class 12 Chapter 3 Electrochemistry Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry 1 Electrochemistry involves the study of the relationship between electrical signals and chemical systems that are incorporated into an electrochemical cell. It plays a very important role in many areas of chemistry, including analysis, thermodynamic studies, synthesis, kinetic measurements, energy conversion, and biological electron transport [ 1 ].
Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
Electrochemistry involves the study of the relationship between electrical signals and chemical systems that are incorporated into an electrochemical cell. It plays a very important role in many areas of chemistry, including analysis, thermodynamic studies, synthesis, kinetic measurements, energy conversion, and biological electron transport [ 1 ]. 5_ Electrochemistry.pdf - Read online for free. Much more than documents. Discover everything Scribd has to offer, including books and audiobooks from major publishers.
Electrochemistry grew out of the same tradition which gave physics the study of electricity and magnetism. The reputed founders of physical chemistry-Arrhenius, Ostwald, and van't Hoff-made many of their contributions in areas which would now be regarded as electrochemistry. With the post-World War II capture of physical chemistry by chemical 01/10/2017 · Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter-relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1. CONDUCTORS AND NON
Electrochemistry Notes Calculating Electrochemical Potential of a Cell 1) Write two ½ reactions written as reductions 2) Compare E’s––the reaction with the highest E is reduced, the other is oxidized Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
Electrochemistry grew out of the same tradition which gave physics the study of electricity and magnetism. The reputed founders of physical chemistry-Arrhenius, Ostwald, and van't Hoff-made many of their contributions in areas which would now be regarded as electrochemistry. With the post-World War II capture of physical chemistry by chemical ELECTROCHEMISTRY Check List Make sure you Can explain how a galvanic and an electrolytic cell works basic Are able to describe the processes and identify the redox reactions that take place in electrolytic and galvanic cells Can explain what happens to current and potential difference when the reactions in a galvanic cell are in chemical equilibrium Are able to describe the standard hydrogen
P a g e 6 6 sat. KCl outer = pH 7 glass membrane in contact with the sample inner glass membrane in contact with KCl Ag wire tip coated with AgCl The pH system (pH cell) has a high internal resistance, therefore the pH meter must have a very high Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element …
Electrochemistry is the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. The subject is of importance both for theoretical and practical considerations. A large number of metals, sodium hydroxide, chlorine, 20/06/2016 · This electrochemistry review video tutorial provides a lot of notes, equations, and formulas that you need to pass your next chemistry test / exam. It’s a nice video that provides an
Ch. 13 Fundamentals of Electrochemistry Yonsei University

Electrochemistry PDF Free PDF Download Notes Problems. Electrochemistry Notes Calculating Electrochemical Potential of a Cell 1) Write two ½ reactions written as reductions 2) Compare E’s––the reaction with the highest E is reduced, the other is oxidized, Introduction to Electrochemistry A.) Introduction: 1.) Electroanalytical Chemistry: group of analytical methods based upon electrical properties of analytes when it is made part of an electrochemical.

ElectrElectrochemistrochemistryy. Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event, Fundamentals of Electrochemistry provides the basic outline of most topics of theoretical and applied electrochemistry for students not yet familiar with this field, as well as an outline of recent and advanced developments in electrochemistry for people who are already dealing with electrochemical problems. The content of this edition is.
E l e c t r o c h e m i s t r y C h 1 9 P a g e 1
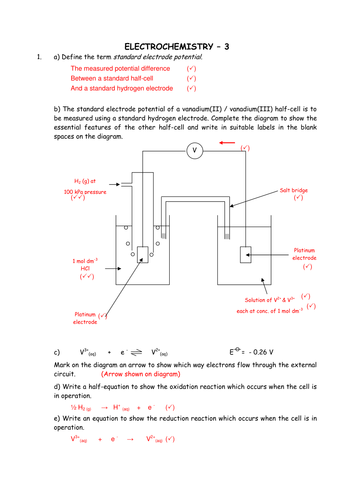
Unit – I Electrochemistry Part – A Questions & Answers. Chemistry Notes for class 12 Chapter 3 Electrochemistry Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry 1 https://en.wikipedia.org/wiki/International_Society_of_Electrochemistry P a g e 6 6 sat. KCl outer = pH 7 glass membrane in contact with the sample inner glass membrane in contact with KCl Ag wire tip coated with AgCl The pH system (pH cell) has a high internal resistance, therefore the pH meter must have a very high.
20/06/2016 · This electrochemistry review video tutorial provides a lot of notes, equations, and formulas that you need to pass your next chemistry test / exam. It’s a nice video that provides an Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by EMAIL (sergio.trasatti@unimi.it) in the format below ie with email and/or internet address. Organisers are asked to submit details at least SIX MONTHS before the date of the event
P a g e 6 6 sat. KCl outer = pH 7 glass membrane in contact with the sample inner glass membrane in contact with KCl Ag wire tip coated with AgCl The pH system (pH cell) has a high internal resistance, therefore the pH meter must have a very high and interdisciplinary nature of electrochemistry, and particularly of electrode reactions, through a description of modern electrochemistry. Secondly to show to the student and the non-specialist that this subject is not separated from the rest of chemistry, and how he or she can use it.
and interdisciplinary nature of electrochemistry, and particularly of electrode reactions, through a description of modern electrochemistry. Secondly to show to the student and the non-specialist that this subject is not separated from the rest of chemistry, and how he or she can use it. Electrochemistry is the study of reactions in which charged particles (ions or electrons) cross the interface between two phases of matter, typically a metallic phase (the electrode) and a conductive solution, or electrolyte. A process of this kind can always be represented as a chemical reaction and is known generally as an electrode
Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter- relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1 . CONDUCTORS AND NON 13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer
01/10/2017 · Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter-relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1. CONDUCTORS AND NON 20/06/2016 · This electrochemistry review video tutorial provides a lot of notes, equations, and formulas that you need to pass your next chemistry test / exam. It’s a nice video that provides an
Unit – I Electrochemistry Part – A Questions & Answers 1. Differentiate between electrolytic cells and galvanic cells? (Nov. 2005), (May 2003) S.No electrolytic cells Electrochemical cell 1 Electrical energy is converted into chemical energy. Electrochemical cell is the one, in which chemical energy is converted into electrical energy. Electrochemistry is the study of reactions in which charged particles (ions or electrons) cross the interface between two phases of matter, typically a metallic phase (the electrode) and a conductive solution, or electrolyte. A process of this kind can always be represented as a chemical reaction and is known generally as an electrode
Fundamentals of Electrochemistry pdf Fundamentals of Electrochemistry pdf : Pages 719 Second Edition by V. S. BAGOTSKY Electric Currents in Ionic Conductors ; Electrode Potentials ; Thermodynamics of Electrochemical Systems ; Mass Transfer in Electrolytes ; Phase Boundaries (Interfaces) Between Miscible Electrolytes ; Polarization of Read and learn for free about the following article: Electrochemistry If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter- relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1 . CONDUCTORS AND NON electrochemistry, beginning with techniques and passing through mechan isms to electroanalytical chemistry, synthesis, industrial electrochemistry, batteries and finally corrosion. All prefaces end with the author thanking a spouse. This is not mere form. Academics with …
20/06/2016 · This electrochemistry review video tutorial provides a lot of notes, equations, and formulas that you need to pass your next chemistry test / exam. It’s a nice video that provides an Chemistry Notes for class 12 Chapter 3 Electrochemistry Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry 1
Electrochemistry involves ionic conduction, the motion of ions through liquid, anions spontaneously move toward the anode and cations spontaneous move toward the cathode. The electrodes are connected externally by a metal wire in which electrons move from the anode (-) to the cathode (+). Metal electrodes are often part of the redox reaction. Electrochemistry involves ionic conduction, the motion of ions through liquid, anions spontaneously move toward the anode and cations spontaneous move toward the cathode. The electrodes are connected externally by a metal wire in which electrons move from the anode (-) to the cathode (+). Metal electrodes are often part of the redox reaction.
Electrochemistry deals with the study of electrical properties of solutions of electrolytes and with the inter- relation of chemical phenomenon and electrical energies. Electrical energy is carried through matter in the form of electric current with the help of suitable source and charge carriers (ions or electrons). 1 . CONDUCTORS AND NON Electrochemistry grew out of the same tradition which gave physics the study of electricity and magnetism. The reputed founders of physical chemistry-Arrhenius, Ostwald, and van't Hoff-made many of their contributions in areas which would now be regarded as electrochemistry. With the post-World War II capture of physical chemistry by chemical